Below is a part of failure analysis report. The analysis was conducted by Doctors J.J.B. Pierre Blais, B.Sc., Ph.D., C.Chem., F.C.I.C. at Innoval, failure analysis report for the 35 year old female implants and capsular.
Innoval is located in Ottawa, Canada, Innoval specializes in the development of health sciences and materials technology education programs as well as the training of paramedical and technical personnel for hospitals, government and industry.
More images below report
Right Implant - patch without evidence of a gel fill hole or seal; patch markings "PIP 285cc 30901 050" appear contiguously under one another. The shell diameter is 126-130 mm. The patch measures 52-53 mm and is placed internally at a shell aperture of 36-37 mm. The approximate projection is 25-27 mm.
Left implant - patch without evidence of a gel fill hole or seal; patch markings "PIP 285cc 30901 056" appear contiguously under one another. The shell diameter is 126-130 mm. The patch measures 52-53 mm and is placed internally at a shell aperture of 36-37 mm. The approximate projection is 25-27 mm.
Observed shell dimenslons for both implants and residual weight correlate with PIP catalogues issued between 1998 and 2002. .,
~
Implant Identification:' The implants are identified by direct inspection as Poly Implant Protheses (PIP) round, textured, "Profil Standard" (low profile) silicone gel-filled mammary prostheses nominally rated at 285 cc, on the basis of the above noted observations. Fabrication peculiarities of each implant are nearly identical suggesting products issuing from the 'same batch. These peculiarities are also consistent with manufacturing techniques employed circa 1998-2002. The key identification attribute is the labeled patch, as described above, without an obvious gel fill hole seal. Secondary identification characteristics include a round, somewhat underfilled-appearing textured shell with a distinctive non-textured circular patch. The texturing
is consistent with a shallow surface-replication process which does not produce tortuous voids or deep pores typical of competing products. The size of the implant shells and the patch markings confirm that the implants were nominally rated at 285 cc. The identification of Ms. XXX implants as Poly Implant Protheses textured silicone gel-filled mammary prostheses as indicated above is definitive. The commercial history of Poly Implant Protheses S.A. and its antecedents is in Appendix A. Catalogue excerpts are in Appendix B.
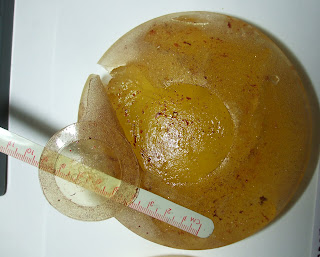
Observed Condition of Right Implant: The implant is received without suspending medium in a knotted, clinically-labeled polyethylene bag. The inside of the bag is coated with a thick layer of invasive oily material. The implant is ruptured with the principal failure line girding the near totality of the patch perimeter, almost separating the patch from the shell. An isthmus of 42-43 mm retains the patch to the remainder of the shell. The rupture line continues radially and crosses the equator, extending into the anterior by about 25 mm. The composite rupture line measures 130-135 mm from end-to-end including the part contiguous to the patch edge. An independent rupture of 50 mm from end-to-end creates a narrow flap/sliver at the equator (see Figure 1 of Appendix C). This zone is approximately 3 mm in width and remains attached to the shell. The equatorial zone is preferentially eroded with loss of texture for about 30% of the circumference. All rupture lines are serrated, indicating progressive shell failure through material fatigue which occurred over many years. The effect is most marked at shell pleats. There is minimal tissue within the shell enclosure. The external shell surface is coarsely textured and the texture is substantially reduced in thickness for the whole surface. The texture extends to the patch edge but the central part of the patch is not textured. Preferential loss of texture is noted at the equator, leaving portions of the shell nearly denuded. The gel is amber in colour and does not adhere to the shell. When placed on a porous medium, the gel separates into two distinct parts with the fluid component being rapidly absorbed within the medium. The gel is cohesive, forming a plug which retains the shape of the implant and maintains all shell parts in their
correct relative position. A thick layer of oil is at the surface of the gel plug. Free oil separates the plug from the inside surface of the implant shell. Bright orange/red matter consistent with degraded blood hemoglobin is intermingled with the free oil and imparts an orange colour to the gel. Overall impressions confirm a primitive breast implant with a weak textured shell filled with a' cohesive oily silicone gel. Mass balance calculations establish that approximately 45 gm of fluid silicone oil-rich material is missing and would have been dispersed into the user's tissue. Conversely, approximately 6-7 gm of the user's body fluid would have been entrapped within the shell enclosure in vivo.
Observed Condition of Left Implant: the implant is received without suspending medium in a clinically- labeled knotted polyethylene bag. The inside of the bag is coated with a thick layer of invasive oily material consistent with low molecular weight silicone oil. The implant is ruptured with a complex failure line which measures approximately 180 mm from end-to-end. A 50 mm central part of the rupture line lies exactly at the patch edge and appears to be the site where shell failure began. Two segments of the failure line project radially to intersect the equator, creating a free flap of shell material which is retained to the anterior side by an isthmus of 70 mm (see Figure 2 'of Appendix C). The failure line is eroded focally, more so at the patch edge and on the equator. Segments of the rupture line are serrated, confirming progressive failure through material fatigue which took place over many years. Finely-divided tissue intermingled with the user's body fluid occupies pools within the shell enclosure. This material has a paste-like consistency and reflects a protracted exposure of the gel to the biological environment. The external shell surface has a coarse texture which is substantially reduced in thickness when compared to unused implants. This focal thinning or-erosion is non-uniform but affects the whole surface. The texture extends to the patch edge. The central part of the patch is not textured. Preferential loss of texture is observed at the equator, leaving some areas nearly denuded. The gel is of amber/orange colour and does not adhere to the shell. When placed on a porous medium, the gel separates into two distinct parts with the fluid part rapidly absorbing into the, medium. The cohesive gel forms a plug which retains implant shape and maintains all shell parts in their appropriate relative position in spite of the ruptures. A thick oily layer covers the surface of the gel plug. Free oil pools separate the plug from the inside surface of the implant shell. Bright orange/red matter consistent with degraded blood hemoglobin mixed with the free silicone oil imparts an overall orange colour to the gel. Collective impressions confirm a substantially identical implant to that of the contralateral side with a cohesive yet degraded gel which easily releases its constituent oils. Mass balance calculations establish that approximately 40 gm of fluid silicone oil-rich material would have been dispersed into the user's tissue over the dwell time. Conversely, about 10 gm of the user's body fluid and tissue would have been entrapped within the shell enclosure in vivo. About 5 gm of this tissue remains within the shell enclosure.
Observed Condition of Right Capsule: The capsule is provided in two separate pieces. The small part, weighing 2.7 gm in the wet state and measuring approximately 50 x 25 x 3 mm, accounts for less than 5% of the theoretical capsule surface. The principal part, weighing 46.1 gm in the wet state and measuring 125 x 100 x 4 mm, has a sacular shape reflecting the spheroidal prosthesis it once contained. This part is irregular in thickness and shows multiple fenestrations with some healed apertures, indicating that the failed prosthesis was not wholly enclosed within capsular tissue (fenestrated capsular enclosure). Collectively, the provided capsular tissue accounts for at least 90% of the theoretical capsule and there is no evidence of sampling of the capsular tissue. However, the jagged and irregular projection of attached external tissue may have been sampled. A noteworthy observation is the large amount of adhering skeletal muscle and fibrofatty material, reflecting a difficult explantation with adhesions to muscle and glandular tissue over large areas.
The inside and outside surfaces of the large segment of capsular tissue are of different morphology and vary in characteristics from point-to-point. The inside surface is mostly smooth and glistening, as expected for a capsule with a long contact time with a prosthetic object. Only small portions of the inside surface show the imprint of the implant texture,. confirming that the texture was not bound into the capsular tissue. Some parts of the interior surface are nodular and cobbled. Mild pressure applied to these areas causes an oily substance to exude from fine pockets in the tissue when a fresh cut is made. This confirms the granulomatous' nature of the capsule and the large uptake of silicone oil derivatives by the capsular tissue. This' uptake is particularly noteworthy in the thicker nodular portions ofthe capsule. The capsule wall is multi-layered and two closed pockets, one measuring approximately 50 x 30 mm with a thickness of at least 5 mm and the other approximately a third of that size, create distinct secondary compartments made up of hyalinized tissue partitions which would have been occupied in vivo by fluid prosthetic material and body fluids (perlprosthetlc seroma and/or hematoma). Embedded coloured material within some of the tissue, consistent with hemosiderin, confirms the presence of small organized hematomas in vivo.
Observed ConditiOn of Right Capsule (cont'd.): Other parts of)the large capsule segment have a rough, cobbled interior and show formation of separate pannus. The pannus is attached to the capsule wall and includes web-like structures and granular fragments of approximately 2 x 2 x 1 mm. These structures suggest ingrowth of capsular tissue into a semi-solid gel mass. The pannus and the related debris consist principally of connective tissue and do not exude oil on compression. Such morphology is not habitually encountered in connection with implant misadventures. The orientation of connective tissue fibres within these areas is also different from what is habitually seen in hyalinized capsular tissue, indicating multiple different and concurrent processes of capsule formation over the long dwell time of the prosthesis.
Thin calcific plaques and their impression at the surface of the inside of the capsule confirm the onset of mineralization (calcification) at the prosthetic interface. The capsular tissue is nearly acellular and cut sections exhibit evidence of minimal inflammatory activity confirming that the inflammatory process had subsided long before. implant removal and that, for practical purposes, the capsular material was not viable tissue (onset of necrosis). The external side 'of the capsule has a large amount of skeletal muscle tenaciously attached. This material is oil-infiltrated and there is evidence of deep inflammation. The amount and the position of this tissue suggest a difficult and laborious explantation with an implant capsule firmly adhering to muscle over an area of at least 20 sq cm. This residual muscle tissue constitutes a reference point for the orientation of the capsule. Residual fibrofatty breast tissue adheres to the opposite pole of the sacular capsule indicating that the capsule simultaneously contacted both the breast gland and the chest muscles. On that basis, it appears that the implant did not lie exclusively within a subrnuscular pocket. Thickness measurements of the. hyalinized zone of the capsule vary widely from point-to-point. The thinnest is approximately 0.1 mm adjacent to fenestrations and the thickest parts exceed 7 mm near the equator of the capsule, the average being 5 mm. Focal discolouration and embedded optically-dense material are visible with transillumination and confirm loci of accumulated hemosiderin and coloured debris.
The small separate part has the same characteristics as the larger portion and its thickness is nearly 5 mm in one part, the average being 4 mm. This part is nodular and focally hemosiderin-laden. Both sides of the tissue coupon show evidence of long term contact with prosthetic material. On that basis, it would appear that this tissue does not originate from the capsule itself but was instead a partition within the gel-flooded space. Its presentation is consistent with pannus or a projection of capsule which separated two loci of gel-infiltrated material.
The gross physical characteristics of the capsule exhibit features which are not frequently encountered in prosthetic capsules surrounding implants made after 1990. These distinctive features are shown graphically in the illustrations of Appendix C. The most remarkable include the large amount of entrapped mobile silicone-based material and the complex multi-compartment character of the capsule with similarities to an abscess divided by internal septa into multiple compartments.
Thin calcific plaques and their impression at the surface of the inside of the capsule confirm the onset of mineralization (calcification) at the prosthetic interface. The capsular tissue is nearly acellular and cut sections exhibit evidence of minimal inflammatory activity confirming that the inflammatory process had subsided long before. implant removal and that, for practical purposes, the capsular material was not viable tissue (onset of necrosis). The external side 'of the capsule has a large amount of skeletal muscle tenaciously attached. This material is oil-infiltrated and there is evidence of deep inflammation. The amount and the position of this tissue suggest a difficult and laborious explantation with an implant capsule firmly adhering to muscle over an area of at least 20 sq cm. This residual muscle tissue constitutes a reference point for the orientation of the capsule. Residual fibrofatty breast tissue adheres to the opposite pole of the sacular capsule indicating that the capsule simultaneously contacted both the breast gland and the chest muscles. On that basis, it appears that the implant did not lie exclusively within a subrnuscular pocket. Thickness measurements of the. hyalinized zone of the capsule vary widely from point-to-point. The thinnest is approximately 0.1 mm adjacent to fenestrations and the thickest parts exceed 7 mm near the equator of the capsule, the average being 5 mm. Focal discolouration and embedded optically-dense material are visible with transillumination and confirm loci of accumulated hemosiderin and coloured debris.
The small separate part has the same characteristics as the larger portion and its thickness is nearly 5 mm in one part, the average being 4 mm. This part is nodular and focally hemosiderin-laden. Both sides of the tissue coupon show evidence of long term contact with prosthetic material. On that basis, it would appear that this tissue does not originate from the capsule itself but was instead a partition within the gel-flooded space. Its presentation is consistent with pannus or a projection of capsule which separated two loci of gel-infiltrated material.
The gross physical characteristics of the capsule exhibit features which are not frequently encountered in prosthetic capsules surrounding implants made after 1990. These distinctive features are shown graphically in the illustrations of Appendix C. The most remarkable include the large amount of entrapped mobile silicone-based material and the complex multi-compartment character of the capsule with similarities to an abscess divided by internal septa into multiple compartments.
Observed Condition of Left Capsule: The capsule is provided in three pieces. The two smallest pieces (specimen "66 A") weigh 7.7 gm and 1.4 gm in the wet state. They measure 50 x 30 x 3 mm and 3 x 2 x 4 mm respectively. Together, they account for less than 10% of the theoretical capsule surface. The principal piece (specimen "66 8") weighs 27.8 gm in the wet state and measures about 130 x 100 x 5 mm. It has a recognizable sac-like shape and is consistent with tissue that would have surrounded a spheroidal prosthesis. This piece is irregular in thickness and shows evidence of sampling by a prior custodian but the sampling did not consume a major portion of the specimen, being limited to slivers of about 1-2 gm each. Collectively, the provided capsular tissue accounts for about 60% of the theoretical capsule. There is a substantial amount of skeletal muscle attached to the large capsule fragment, reflecting deep adhesions to muscle and/or chest wall but the amount is less than on the contralateral tissue specimen from the right breast. A small amount of fibrofatty tissue is also present, confirming that the implant was not wholly within the submuscular compartment.
The inside and outside surfaces of the large segment of capsular tissue are widely different in presentation and the morphology varies frompoint-to-point. The inside surface is smooth and glistening for about half of the total surface. The other half is rough, nodular and exudes oil on mild compression. The rough part also has projecting connective tissue and material of nodular appearance. This is the thickest part of the specimen (approximately 2-4 mm). The inside surface does not show the imprint of the implant texture, confirming that the intracapsular space was flooded with fluid and that the prosthesis surface did not consistently contact the capsule wall. Some areas of the interior surface are cobbled with projecting connective tissue. This unusual material appears to have formed through connective tissue ingrowth in a semi-fluid mass, most probably extravasated gel. Mild pressure applied to the capsular material causes an oily substance to exude from fine pockets in the tissue. There are no large pockets of oil. Instead, the oily material is distributed throughout the tissue, indicating that most of the capsule has a granulomatous character and that. there was a large uptake of silicone oil derivatives. An approximate 6 sq cm area shows smooth and mildly nodular capsular material on both sides, confirming that extravasated prosthetic material contacted both the inside and outside surfaces of the capsule. Such an observation establishes that the capsule had a multi-compartment character, most probably through folding of the implant or gross extravasation and accumulation of oily fluid.
The large capsule segment has no separate compartment, no organized hematoma or other irregularity associated with compartmentalization. The thicker portions of the capsule are remarkable for vascularization with blood vessels as large as 1 mm distributed over an area of about 3 sq cm. The -btood vessels occupy the outside surface of the capsule and are limited to the thick nodular tissue parts. These areas also show a small number of viable cells, indicating that, on average, the left capsule suffered less chemical insult than the tissue of the contralateral right capsule.
The two small capsule fragments have substantially the same structure of the large piece, however, the hyalinized zone is much thicker, averaging approximately 4 mm. Adhering skeletal muscle is also found over an area of approximately 1 sq cm confirming that at least one part of the capsule adhered strongly to muscle and a substantial portion of the muscle was resected to free the adhesions. For practical purposes, the smaller segments can be considered as material of the same origin, most probably separated from the main capsule during explantation or the pathology study.
Overall impressions suggest that the capsule developed in a stagewise process over many years and that the stages formed unique parts of the structure at different times. There is no calcific plaque or evidence of mineralization on any part of the provided capsule suggesting that the rupture of the left implant is more recent than for the right implant. The ongoing inflammatory activity, limited to. the left side, also suggests that the capsule was still in a development stage at the time of explantation and that the inflammatory process was maintained.
Discussion - Clinical Aspects: In June 2002, Ms. XXX underwent cosmetic mammoplasty with insertion of Poly Implant Protheses S.A. (PIP) round, textured, silicone gel-filled mammary prostheses of low profile ("profil standard"). The implants had a nominal rating of 285 cc and were placed in the submuscular position against the chest wall. Textured prostheses pose problems which are not encountered with smooth-walled implants. Textured surfaces are sources of finely-divided debris which detaches from the prosthetic surface. The texture also contributes to formation of complex multi-compartment capsules. Lastly, when placed in direct contact with the chest wall, the texture enhances inflammation and irritation, culminating in adhesions. These issues are discussed Appendix D. The submuscular position magnifies the risk of adverse events and makes explantation more difficult and damaging. In the event of rupture, extravasated substances invade a broader area and affect the lymphatic system more severely. Aspects relating to submuscular placement of mammary implants are discussed in Appendix E.
Ms. XXX encountered immediate complications following implantation with severe pain extending for more than one week. Shortly after, one of th~ implants migrated from its original pocket and a portion bulged, causing an externally-visible anomaly. Such an observation suggests that the implant was not wholly covered by the muscle and that the prosthesis migrated away from the pectoralis muscle, compressing one part and allowing the other part to bulge prominently.
In 2009, Ms. XXX had a successful but difficult pregnancy. Breast infection (mastitis) was encountered during breastfeeding, requiring antibiotic treatment. Breastfeeding is a problem-riddled practice for breast implant users, as discussed in Appendix F. Moreover, major changes occur in the breast structure during breast engorgement, further contributing to stresses on the prostheses and capsules. By late 2010, tremors and neuromuscular disturbances were being experienced. Additional symptoms appeared during November/December 2010, including severe chest pain, skin sores, hair loss, rash with discoloration and tingling of extremities. Unusual sensations in the breast and rib cage as well as mouth ulcers were subsequently experienced. Diagnostic tests were conflicting. Symptoms worsened during late 2011. A CT-scan of the chest was performed in August, 2011 but the significance of the images' may not have been understood. On January 3, 2012, the implants and their associated capsular tissue were removed.
Discussion - Findings from the Innoval Study: On January 5; 2012, Ms. XXX pathological material was received at lnnoval. It consisted of two silicone gel-filled breast implants and capsular tissue from the right stde. Capsular tissue from the left side was received on January 17, 2012. Examination of the implants confirmed PIP products with manufacturing attributes typical of PIP prostheses made circa 1998- 2002. Both implants showed large ruptures initiated at the boundary of the patch and the shell (see Figures 1 and 2 of Appendix C). The failure lines continued radially, intersecting and projecting beyond the equator. This mode of failure is uncommon. Worse, the peculiar properties of the gel core with its ability to exude oil allowed detachment of the gel core from the shell, creating a space between the core and the shell. The space refilled with body fluid and tissue. Ruptures initiated at the patch edge indicate poor shell material, vulnerable to fatigue and fragmentation under mild stress. Simultaneously, compression incidental to user movement, in particular from arm and chest muscle activity, increases the destructive effects.
Combined effects from stresses and capsular contracture would have introduced additional forces on the prostheses. Breast implants with a low profile (,Profil Standard') are subject to severe creasing and folding. A fold near or across the patch enhances the risk of early rupture in that vicinity. The PIP implants in question embodied the worst contributing factors, specifically a pleat-inducing configuration, poor shell materials, a susceptibility to adhesions to surrounding muscle and a vulnerability to stresses from movement. Conversely, implants with a more prominent profile approximating a spheroidal object are more resistant. However, poor shell materials and a flawed patch-making technique are decisive deficiencies and early rupture is the expected outcome.
Discussion - Findings from the Innoval Study (cont'd.): All breast implants induce formation of a tissue capsule which consists of a thin layer of connective tissue, termed 'hyalinized zone'. This zone is composed mostly of collagen fibres oriented parallel to the implant surface. At the outset, the capsule is permeable to water-rich fluids and is moderately strong. With time, shrinkage causes contracture. For implants which have minimal inflammatory effluents and a reasonable degree of quality, the capsular layer is less than 0.3 mm and remains that way until other factors cause it to thicken and change in composition. Many breast implants do not fulfill basic requirements of design and quality. Predictably, they induce abnormal capsules. Implants that degrade and fail with release of solid debris and oily reactive fluids rapidly converge to an abnormal state, culminating in structures where the prosthetic impurities become an integral part of the capsule. The interaction between the contaminated capsular tissue and natural physiologic processes adds another dimension to the problems breast implants engender. This is further discussed in Appendix G. Capsules also add to mechanical stresses acting on implants but these are minor unless there is a large amount of abnormal 'tissue distributed asymmetrically around the implant. The role of the capsule in implant injury is discussed in Appendix H. All of these factors contributed to failure of Ms. XXX implants and her present condition.
Ms. XXX retained her PIP implants for approximately 10 years, considerably longer than the typical PIP user. Both of her capsules were. very thick, markedly contracted and adhered broadly to muscles. The problem was more pronounced on the right side. Adhesion between capsules and muscle tissue is not rare but drastic adhesion with integration of the muscle into adjacent structures over a large surface is infrequent. The adhesion, in combination with capsular contracture from the strong, thick capsular tissue, caused both of Ms. XXX prostheses to fold and crease sharply, more so on the right side. As the tissue thickened, the capsules locked the implants in a permanently folded configuration. The compressive folding increased the destructive forces on the implants near the patch.
Examination of the failure lines on both implants showed the ruptures to be of longstanding origin. The failure edges were worn and rounded with loss of texture on some parts. These observations established that the implants lost the-ir integrity within 2-3 years after implantation, the right implant Jailing first. Early shell failure from material fatigue is a marker for incorrectly-formulated and improperly-processed shells. Rapid loss of texture is also a symptom of faulty manufacturing. This suggests major problems at the manufacturing plant. Examination of other PIP implant shells made within the same period as Ms. XXX prostheses show similar features and nearly identical failure modes. Patch edge failure accounts for the majority of PIP failures observed to date.The filling gel contributes to the injurious potential of an implant. Ideally, the gel should be biologically and chemically inert and should not release mobile reactive substances into adjacent tissue. In practice, this is rarely the case. If manufacturing technology is poor and quality assurance practices are lax, the fraction of faulty implants within each batch increases drastically. With a near certainty of shell rupture and substandard filling materials, the risk of injury from the implant is not a possibility but a certainty and the damage increases with implant size and dwell time. In that context, Ms. XXX implants met all conditions to cause severe and early injury.
The gel in Ms. XXX implants did not flow easily and consisted of two distinct and easily separable components. The oily component effused rapidly. Measurements demonstrated easy separation of at least 20% of the gel mass which reappeared as a mobile oil rapidly absorbed within porous media and tissue. Moreover, this oily gel component could be easily dispersed in aqueous media to produce a low viscosity emulsion that would have been entrained in the user's body fluids. The gel could be easily fractured into smaller pieces through mild compression, making the oily component even more easily released. Fluid oily gel with easily-separable oils were common in breast prostheses of the seventies and eighties. Cohesive gels which exuded their oily components also existed during the sixties and became fashionable again in the early nineties. Thus, Ms. XXX implants could be perceived as products manufactured according to recipes, processes and standards used two and three decades ago. Similarly, the adverse effects she suffered are similar to the problems encountered by users of breast prostheses made during the earlier decades. However, Ms. XXX adverse effects were more intense, possibly because of the unusual contaminants within the gel mixture.
Discussion - Findings from the Innoval Study (cont'd.): Examination of Ms. XXX capsular tissue revealed a complex interface between the implants and surrounding structures. There were multiple layers of hyalinized tissue forming distinct fluid-filled compartments. The right side was particularly compartmentalized with three pockets of substantial size filled with oils, body fluids and emulsified substances that comprised shell, gel and tissue debris. Body fluids also infiltrated both ruptured shells and filled the space between the shells and the gel cores, forming additional pockets. These sites would have resembled compound abscesses with walls and trabeculations flooded by reactive biological fluids and prosthetic by-products. Mass balance measurements on the implants established that about 40 grams of gel were missing from each. In vivo, most of this material would have consisted of the oily gel part and would have been in the fluid occupying the abscess-like cavities. The wall of these abscess-like structures exceeded the normal capsule thickness by almost 30 times. Such a thick wall would have limited fluid exchange between the abscess cores and the outside of the abscesses. The stagnant conditions would have accelerated tissue dehydration following the initial inflammatory reaction to the prosthetic impurities.
Discussion - Findings from the Innoval Study (cont'd.): Examination of Ms. XXX capsular tissue revealed a complex interface between the implants and surrounding structures. There were multiple layers of hyalinized tissue forming distinct fluid-filled compartments. The right side was particularly compartmentalized with three pockets of substantial size filled with oils, body fluids and emulsified substances that comprised shell, gel and tissue debris. Body fluids also infiltrated both ruptured shells and filled the space between the shells and the gel cores, forming additional pockets. These sites would have resembled compound abscesses with walls and trabeculations flooded by reactive biological fluids and prosthetic by-products. Mass balance measurements on the implants established that about 40 grams of gel were missing from each. In vivo, most of this material would have consisted of the oily gel part and would have been in the fluid occupying the abscess-like cavities. The wall of these abscess-like structures exceeded the normal capsule thickness by almost 30 times. Such a thick wall would have limited fluid exchange between the abscess cores and the outside of the abscesses. The stagnant conditions would have accelerated tissue dehydration following the initial inflammatory reaction to the prosthetic impurities.
The inflammatory reaction evidently subsided in the late phase. At the time the implants were explanted, active inflammation had almost ceased on the right side because of depletion of viable cells. Low grade inflammation was still present on the left side. Necrosis of the tissue affected most of the capsular material. Calcification had begun on the right side, confirming an advanced degree of tissue denaturation. The structure of the abscess-like implant pocket is illustrated in Figure 3 of Appendix C. These observations suggest that the patient no longer had the capacity to react against the invasive material and that the bulk of the tissue surrounding the failed implants was, for practical purposes, a non-viable, partly-denatured composite material with a large fraction of synthetics;
Retrospective review of low resolution copies of radiographic projections from a CT scan of Ms. XXX chest performed in August, 2011 revealed an implant in direct contact with bony tissue on the chest wall. This same implant demonstrated frank shell disruption on the anterior with deep infiltration of aqueous fluids within the gel mass. Such findings confirm a major rupture with broad dispersal of gel. The projections also showed severe spherical contracture around the failed implant and an unusually thick capsule. The contralateral implant was not clearly visualized.
Discussion - Long Term Health Impact: Mammary implants injure in different ways and their impact varies with user-susceptibility, implant size, type, position and dwell time. Other factors can be involved if there are complications such as a protracted delay in removing a failed implant. Mechanisms of injury are discussed in Appendix I.
Ms. XXX experience with her PIP silicone gel-filled implants cannot be compared to a simple implant failure resulting in temporary breast deformity and discomfort. There is a severe penalty from breast implant failure when surrounding tissue is inflamed with formation of granulomata and tissue necrosis. Such abnormal tissue must be removed otherwise severe side effects including incomplete healing of the surgical site result. Prosthetic residuals from implants can also be dispersed within body structures, causing late adverse reactions with fibrosis and secondary calcification. Systemic sequelae may take place in sensitive individuals. Even with complete debridement of visibly-contaminated tissue and prosthetic residuals, remnants of foreign debris and denatured tissue can remain and may need to be selectively removed through supplemental surgery. It is rare for users of silicone or saline-filled implants to suffer muscle destruction, adhesions and tissue inflammation to the degree encountered by Ms. XXX. Explantation and debridement secondary to implant failure rarely result in damage to the chest wall, as took place for Ms. XXX because of the unusually long delay in removing the failed extravasated implants. Typical implants do not release large quantities of inflammatory.substances that intermingle throughout the breast and chest, as also occurred for Ms. XXX. Formation of large multi-compartment abscess-like structures is comparatively rare, in particular for individuals with an implant dwell time of about 10 years. Abscess-like conditions lead to systemic disturbances adding to the complexity of the injurious process. This is discussed in Appendix J.
Conclusions: The PIP silicone gel-filled implant was ill-conceived from the outset. It had a textured shell prone to adhesion to adjacent tissue and likely to worsen inflammatory reactions. Its low profile configuration made it susceptible to deep pleating and early failure. The patch bonding method altered the mechanical properties and the resistance of the shell at the point of contact. This explains in part why PIP implant shells habitually fail near the patch edge, as did Ms. XXX. The PIP shell was not optimized for individual filling substances and PIP employed the same shell for all of its implants, be it silicone gel-filled, saline filled, polyurethane foam-coated, etc. For silicone gel-filled applications, the shell and gel compositions were inconsistent from batch-to-batch. Independently of batch, the gel contained separable low viscosity invasive oils which acted as carriers for chemical impurities
habitually found in prosthetic gels. Other impurities were introduced from the poorly-selected intermediates used without purification. Post-fabrication cleaning of shells was evidently not performed or incorrectly conducted, leaving the original impurities on the surface. The sterilization treatment, based on gamma radiation, altered the .propqrties of the shell and the gel in unpredictable ways and many aspects of fabrication were without significant quality assurance review. In summary, users of such prostheses would have no probability of retaining the products for more than a few years without sustaining injury.
As manufactured, typical PIP implants did not have the ability to retain adequate physical properties for more than a few months. Shell failure was probable after about 24-36 months. For Ms. XXX, when the shells failed at the patch boundary, the gel was exposed to tissue and body fluids and the oily additives were absorbed by the surrounding tissue. Inflammation caused build-up of fluid within the implant pocket, creating an abscess-like condition which was maintained for at least 6-7 years. During that time, the proliferative capsular tissue bonded deeply within adjacent muscles, ensuring that user movement would cause more oil and impurities to be released. This process maintained the inflammatory activity until most of the surrounding tissue ceased to be viable. Calcification then set in. Distal migration of oil-soluble impurities and uptake of these substances by fatty tissue and the breast glands would have also occurred early. These phenomena were easily predictable on simple physico-chemical and physiologic considerations.
Disclosures in PIP literature were grossly at variance with established facts including the inevitability of early shell failure and its inflammatory sequelae. In spite of repeated consultations, Ms. XXX was not advised of the risks in retaining her implants in a ruptured, partly extravasated condition. The protracted exposure to aggressive substances destroyed essential structures within her chest. The eventual explantation proved difficult and destructive as deep resection of tissue including chest muscles was necessary. Continuing sustained inflammatory reaction from residuals in sites remote from the implant pockets will necessitate a protracted healing period, possibly with supplemental focal
debridement of accumulated pockets of oily substances.
Health history, another part of the report can be found HERE







No comments:
Post a Comment