Is it Hypocritical or Hippocratic oath?
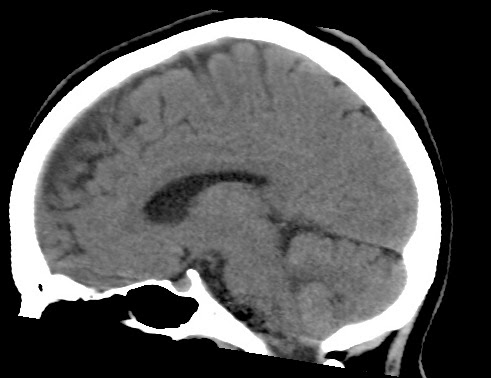
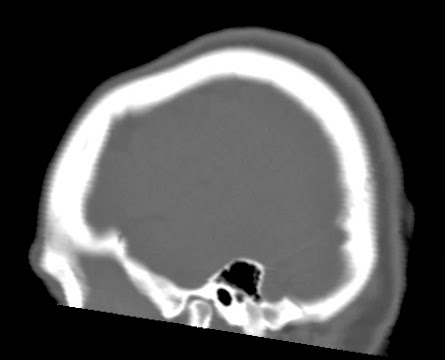
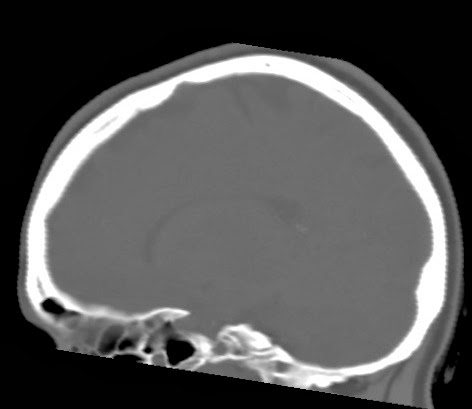
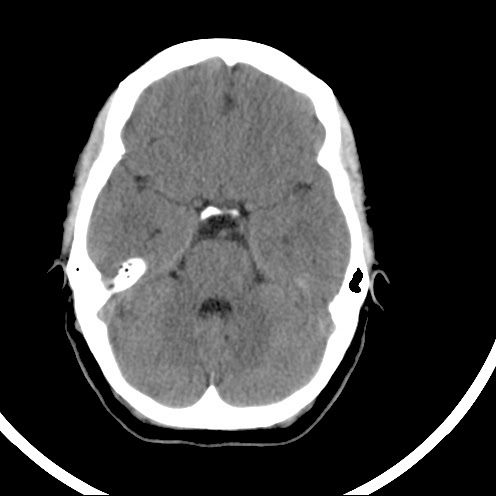
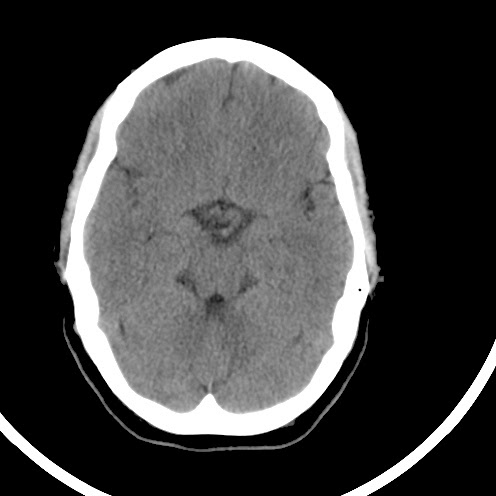
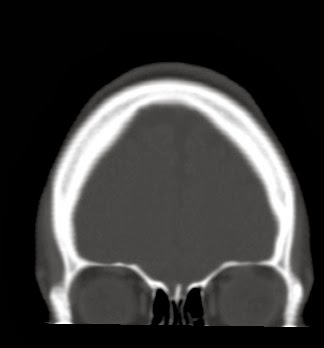
This web-page is dedicated to women with breast implants and their children. You may find radiology images and symptom information. Also images related to physical symptoms taken by the patient himself. Click on images for a larger view. At the moment all patients are from Iceland. The medical and radiological examination conducted at the time did not lead to diagnose. For larger view - click on image.
Comments
Please don't hesitate comment. All ideas and thoughts are highly appreciated. It is open for anonymous feedbacks if preferred.
Thursday, September 11, 2014
Friday, August 15, 2014
BREASTFEEDING AND PROSTHESES
Innoval Failure Analysis Ottawa, Canada K2A 2V1 February 2008
Breast prostheses have been implanted in large numbers for more than forty years. Users have ranged from late adolescence to individuals of advanced age. The socio-economic profile of this cohort is tilted towards individuals with an age range of 25-40. Fertility and lactation for such a group would not normally be a dominant factor. The small fraction of users undergoing reconstruction after tumor resection or fibrocystic mastectomies need not be concerned with breast feeding. On average, users are in the late fertility phase, are parous and do not plan additional pregnancies. However, there is a younger sub-group consisting of women who hope to remain fertile for several years post-implantation and many expect later pregnancies. The concerns with breast feeding applies primarily to this sub-group. With findings of the eighties and nineties regarding long term adverse reactions from implants, there is a rising population of individuals who have had breast prostheses removed electively or following complications. Pregnancies are found within this sub-group. Breast feeding is therefore a consideration for a significant fraction of current and past breast implant users.
Implantation of a large foreign object in the breast requires a major modification of the breast structure. Upon removal of the prosthesis and even under ideal conditions, the structure and the physiology of the gland are not restored to their original state: Thus, the very act of inserting such an object at that location has lasting and irreversible consequences, all of which militate strongly against successful breast feeding. The changes, both normal and pathological, greatly increase the probability of complications to the user as the breast engorges and tissue is deformed. Concurrent physiologic and chemical changes in the breast area further impact adversely on the amount and quality of the milk produced in such lactating individuals.
The origin of current beliefs suggesting that breast feeding is possible and safe for implant users is not known. What is known is that in the early-sixties and seventies, breast feeding was not deemed desirable. Some physiologists did not regard it as generally possible. Their views were based on biomechanical, biochemical and physiologic considerations. Ttie prostheses incorporated mechanically damaging features such as fibrosis-inducing appendages which were expected to modify the breast and eventually damage the vasculature and the lactation apparatus over the long term. The high impurity levels, in particular the oils similar to what had been used before in connection with silicone-injected breasts, were also factors deemed to impact adversely on lactation for toxicological reasons. At any rate, the experience of oil-injected patients had produced sufficient pathological information to support the view .. In the years that followed, the issue remained dormant and no major investigation was performed. Instead, a combination of hearsay and fiction enveloped the area and it gradually became accepted amongst the lay public and some general practitioners that cosmetically augmented patients would routinely be able to breast feed without problems.
This optimistic outlook is not shared by individuals close to the field. The emergence of foam- coated implants in the seventies further reinforced the basis for contra-indications. The dissent came from members of the mainstream medical community fluent with breast prosthetics technology and included pioneer plastic surgeons. However, their views were not widely publicized. The aggressive promotion of cosmetic augmentation started in the late seventies submerged conservative opinions based on pathology studies. Chemical debris from filling materials and unstable coatings of unknown composition, as well as the frequent infections around implants, were the dominant factors supporting the opinions.Fertility and lactation studies were nev,~erconducted systematically by opponents or proponents. In retrospect, this is not surprising because the work seemed unnecessary. Collapse of the lactation system was a logical clinical expectation from gross contamination of the breast by dispersible reactive debris. Similar results were also expected from the introduction of implants that exerted continuous pressure on the breast gland, the vasculature and, in addition, caused uncontrollable fibrosis. Other concerns focussed on fibrosis and the impact of fixation appendages. These accessories altered the breast and removed much of their elasticity. This would have induced severe discomfort upon engorgement prior to lactation.In the late-seventies, the popularity of the procedure increased significantly and large numbers of low quality prostheses were being implanted. Adverse reactions, failures and compatibility problems were widely encountered by the plastic surgeons and the industry but the information remained within that community. The mainstream medical community believed that complications amongst implant users were rare because publications reflected only favorable results. Poor inter-professional communication gave further credibility to the misconceptions. Adverse reactions were being encountered and many of the problems had clear impact on the ability of implant users to breast feed safely and without risk to the infants.
European publications of the seventies contra-indicated breast feeding. Their views were based on empirical data gathered from users of the previous decade as well as less prominently cited research from a few centers, in particular Switzerland. Contra-indications to breast feeding focused on retention of desirable cosmetic results (avoidance of post-lactation ptosis and tissue distention), comfort and absence of pain in the augmented breast (stretching and pressure in the breast structure during lactation), microbiological colonization of the implant area (intracapsular mastitis) and toxicological considerations from implant materials.Biomechanical and physiologic considerations of the prosthetically. modified breast clearly demonstrate why breast feeding is impractical and frequently destructive. The use of a retromammary implant eventually causes pressure atrophy of the breast gland and collapse of the ducts which convey fluid to the nipple. It is very rare when atrophy is not present in surgically augmented breasts after implants have been in site for 2-4 years. Microvascularization within the critical area is drastically reduced and prominent veins have appeared in the periphery of the breast implant close to the skin. In some cases, compression also causes focal ischemia and oxygen depletion. These factors militate towards failure of the milk producing system.
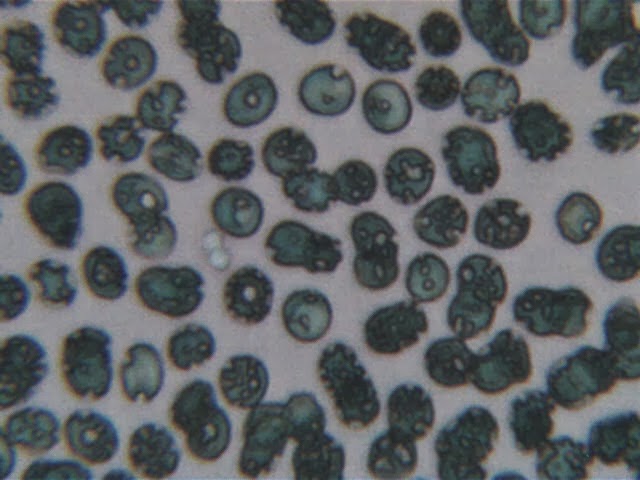
Assuming a successful pregnancy takes place and the breasts engorge in preparation for breast feeding, additional compression results and in some instances pain will be noted, thus making the process uncomfortable and unpleasant. Upon cessation of breast feeding, the breast returns to its initial augmented volume but tissues and ligaments are stretched and the patient re- encounters ptosis. Thus, the cosmetic benefits may be lost thus invalidating the rationale for the implantation. Re-do surgery often takes place following difficult lactation.Breast prostheses are not conventional medical products; they lack many of the attributes of other classes of implants. Many were little more than novelty items. Throughout the years they have been a heterogeneous mixture of-'j!lenerally low quality products with high levels of impurities and features which made their users vulnerable to chronic infective processes. Like most implants, they could induce infections that would remain dormant for many years but still produce destructive local effects and high quantities of microbiological metabolites. The capsule around the implant rarely sealed the compartment. It only delayed the onset of release. Capsules deteriorated and remodelled with time. They eventually opened to release their content some time in the 4th-8th year of post-implantation or after trauma to the chest. Even common contracture treatments fostered infective complications.Processes such as compression capsulotomies and intentional percutaneous perforation of the outer envelope on double lumen implants (two widely used procedures in breast augmentation practices) encouraged colonization and release of microbiological material to the lactation apparatus. The procedures would release large amounts of impurities with unknown biological effects. Chemical impurities included important quantities of substances that varied from brand to brand and frequently from batch to batch of the same brand. The impurities were not limited to silicone oils; they included substances as diverse as platinum organometallics (alkylating agents) as well as residual solvents, reactive silicone intermediates, silica filler, in addition to biologics and denatured tissue arising from biological and microbiological activity within the capsular area. Sub-mammary implant locations gave direct access of the breast gland to prosthetic impurities and contaminants.Implantations in the sub-muscular position were no better. The continuous mastication of the devices by muscle movement accelerated the release of debris from the implants and led to comparable levels of contamination within the general upper chest area. Evidence of necrosis and tissue degeneration surrounding implants is found in nearly all users with implant dwell times exceeding three years. Graphic evidence of this is seen from mammographic studies showing large quantities of calcific debris associated with tissue deterioration and fat necrosis.Multi-lumen and saline implants were potentially much worse. The multi-lumen devices were more prone to contaminating the breast with micro-organisms and their metabolites. They also dispersed finely divided silicone oil emulsions and silicone related by-products within the saline compartment. Saline-filled devices had unstable shells which degraded rapidly to expose the silica filler; this material spallated and invaded tissue. It had an ability to stimulate the production of deviant biologics from the patient's own proteins and tissues. The saline charge in these devices could not be maintained sterile. The compartments habitually contaminated early with innoculae of atypical organisms. They in turn added their toxins to the already substantial list of implant-related debris which had access to the breast compartment.
Paradoxically, national and international parenthood and breast feeding promotion organizations maintained that lactation in implanted mothers was feasible, practical and safe. Today there are still organizations who support the practice on the strength of opinions devoid of clinical, physiologic and biochemical basis. To this day, there is no scientific basis to assume safety for the lactating mothers with implants or their offspring. On the contrary, established thinking based on the physiology of the breast strongly supports an elevated probability of adverse effects. Preliminary studies conducted on small samples of children from implanted mothers note adverse effects and should be a strong deterrent to breast feeding for any individual who either has or who has had a breast prosthesis in her lifetime"lie In the light of present knowledge, a firm contra-indication is still justified on breast feeding for individuals with implants in situ. The contra-indication is as important for the implant user as it is for the infant. For individuals who have had implants which have been subsequently removed as a result of complications, the recommendation is equally valid. Infective sequelae are frequently followed by longlasting manifestations which may produce large quantities of micro-organisms and there by-products may enter the ductal system to ultimately alter the quality of the milk and possibly affect the infant. Individuals with a history of problems or with evidence of hematomas, seromas or nipple discharge should be strongly advised against breast feeding. In this instance, the risk may be greater to the offspring than to the mother.
Innoval Failure Analysis Ottawa, Canada K2A 2V1 February 2008
Thursday, August 14, 2014
THE PIP DEBACLE OF 2012
Emerging Problem -
Medical services relating to adverse events from breast implants have continually increased, with attendant costs. Demand for such services will reach a crescendo as users of the controversial PIP implants undergo explantation on recommendation of national regulatory bodies. The method of implant removal is critical, not only to the long term health of the patient but to the sustainability of publicly-funded implant removal programs. Explantation must be performed by surgeons with the skill and experience to correctly remove the implants, their debris and the contaminated surrounding tissue.
A limited number of surgeons worldwide have such attributes and they will be unable to meet the demand. Individuals with failed implants are generally unsuited for implant replacement. If re-implanted, the cycle of adverse events will be repeated, in particular if the new implants are inserted into contaminated, undebrided implant pockets. Guidelines with oversight provisions must be established to ensure that the morbidity from past abuses does not continue or worsen ..Background - Poly Implant Protheses (PIP) is a France-based company near Toulon.
PIP was founded by Jean Claude Mas in 1991 and consolidated assets from several bankrupt breast implant firms. PIP has a long history of regulatory infraction worldwide and again became topical in 2010 when it declared judicial bankruptcy. French authorities investigated PIP in the context of commercial fraud, money laundering and user injury. The situation was complicated by the deaths of several officials connected with PIP and its funding agencies. The injurious potential of implanted PIP products will have repercussions on healthcare services for many years to come. This briefing addresses aspects of healthcare needs secondary to the removal of PIP and substantially similar mammary implants deemed dangerous and eligible for removal under provisions of healthcare programs.
Rationale for Action -
Corporate records establish that PIP and its precursors made more than 500,000 breast implants between 1989-2010. At least 60 countries received shipments of PIP implants. The implants were also distributed through clearing houses and via internet commerce. Based on direct examination of a significant number of explanted PIP prostheses, they did not fulfill basic safety requirements. The level of risk varies depending on the type and year of manufacture but there is no mechanism to assess which type or batch of PIP implants constitute the most acute risk. Additional concerns include product cleanliness and sterility, the haphazard and undocumented use of intermediates intended for industrial applications, falsified expiry dates, product mislabeling and compromised packaging
.Implant Recall is Problematic -
The recall of an unused implant poses minimal logistics problems and retrieval of outstanding stock can be comprehensive. However, if the product line has been in commerce for a long time, retrieval is nearly impossible as many will have been implanted. Patient follow-up then becomes key. Patient tracking is historically poor in the breast implant trades and a significant number of users will be inaccessible because of death, geographic moves or peculiarities of lifestyle. If the recall is widely publicized, a sizeable fraction of users may come forward and the decision to seek explantation becomes a personal choice. In the case of the PIP 'recall', it is probable that the overwhelming number of users will opt for explantation. Logistics of Breast Implant Removal - The removal of an implant requires a health care provider, typically a surgeon.
However, the quality of the outcome and long term consequences of the failed implantation will vary drastically depending on the surgeon's skill, experience and commitment. Incorrectly-performed explantation can leave the patient with severe and lasting adverse consequences. There is an established tradition for surgeons not engaged in plastic surgery to avoid patients who encounter complications from cosmetic surgery. Thus, individuals seeking explantation are referred back to their plastic surgeon. It is another tradition that many cosmetic surgeons are inclined to replace implants with minimal surgical debridement.
Given the number of users who may seek explantation as a result of the publicity about the PIP incident, the most' competent surgeon's -will develop prohibitively long waiting lists. Even more probable, is that marginally qualified health care providers will be pressed into service.The Technology of Implant Removal - Whereas the insertion of mammary implants is a simple task that may require as little as 20 minutes and rarely more than 60 minutes, comprehensive implant removal and tissue debridement may demand in excess of 150 minutes.
When an implant has been in place for more than a year, an enclosure of specialized tissue, termed 'capsule', forms to insulate the chest structures from the foreign substance. At explantation, this capsule must be removed to avoid long term complications secondary to a failed implant. For implants in situ for more than five years, the capsule will of substantial thickness and willoften adhere tenaciously to chest muscles. Thus, explantation must include the simultaneous removal of the implant with its surrounding capsule, preferably as if it were a single, tumor- like entity. This procedure, termed 'extracapsular capsulectomy' or 'excerese en bloc', is the standard of care. However, it is a demanding task on the part of the surgeon and requires expertise. The number of surgeons with such experience is limited. Conversely, the removal of an implant without capsule removal is propitious for recurrent and more severe symptoms.
Expected Condition of Removed PIP Implants - PIP implants were optimized for minimal cost. With this perspective, shells were used for multiple applications. For example, implant shells filled with saline, hydrogel or silicone gel were considered interchangeable. Shell materials have different performance characteristics depending on their content.
Silicone gel-filled PIP implants employed a penetrating and strongly-interacting diluent in the gel. This diluent altered shell durability, resulting in statistically-elevated early failure rates. PIP implants filled with water-based hydrogels and saline also had poor durability characteristics.
In essence, after a dwell time of about five years, the near totality of PIP shells will have sustained rupture. Under these conditions, implant removal will present as more laborious and lengthy. Complications are also more likely, in particular for the silicone gel-filled PIP implants which contain aggressive intermediates that impact adversely on tissue. When gel contacts tissue, an inflammatory reaction sets in. With time, a composite of tissue containing finely-divided gel pockets and reactive prosthetic intermediates forms. Termed 'granuloma', these entities, which present as rubbery, reactive lumps of altered tissue, cannot be left behind as they behave as aggressive implant-like substances. Granuloma or 'granulomata' must therefore be resected to ensure a reasonable probability of returning the chest to as near a normal condition as possible. Muscle destroyed by the implant effluents or damaged during the debridement surgery must also be repaired. These considerations further increase demands on the surgical team.
Innoval Failure Analysis Ottawa, Canada K2A 2V1 January 2012
Sunday, February 23, 2014
Thursday, January 16, 2014
Subscribe to:
Posts (Atom)